On the Law of Distribution of Energy in the Normal Spectrum
Max Planck
Annalen der Physik
vol. 4, p. 553 ff (1901)
[Transcriber's Note: Where an equation is numbered and NOT part of
an image, I have used a pound (#) sign to indicate a numbered equation.
Due to the formatting, I would not want some to think that a numerial
factor was being used in the equation. J.P.]
The recent spectral measurements made by O. Lummer and E. Pringsheim1, and even more notable those by H. Rubens and F. Kurlbaum2, which together confirmed an earlier result obtained by H. Beckmann,3
show that the law of energy distribution in the normal spectrum, first
derived by W. Wien from molecular-kinetic considerations and later by
me from the theory of electromagnetic radiation, is not valid
generally.
In any case the theory requires a correction, and I shall
attempt in the following to accomplish this on the basis of the theory
of electromagnetic radiation which I developed. For this purpose it
will be necessary first to find in the set of conditions leading to
Wien's energy distribution law that term which can be changed;
thereafter it will be a matter of removing this term from the set and
making an appropriate substitution for it.
In my last article4 I showed that the physical
foundations of the electromagnetic radiation theory, including the
hypothesis of "natural radiation," withstand the most severe criticism;
and since to my knowledge there are no errors in the calculations, the
principle persists that the law of energy distribution in the normal
spectrum is completely determined when one succeeds in calculating the
entropy S of an irradiated, monochromatic, vibrating resonator as a
function of its vibrational energy U. Since one then obtains, from the
relationship dS/dU = 1/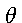 , the dependence of the energy U on the temperature
, the dependence of the energy U on the temperature 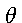 , and since the energy is also related to the density of radiation at the corresponding frequency by a simple relation,5
one also obtains the dependence of this density of radiation on the
temperature. The normal energy distribution is then the one in which
the radiation densities of all different frequencies have the same
temperature.
, and since the energy is also related to the density of radiation at the corresponding frequency by a simple relation,5
one also obtains the dependence of this density of radiation on the
temperature. The normal energy distribution is then the one in which
the radiation densities of all different frequencies have the same
temperature.
Consequently, the entire problem is reduced to determining S as
a function of U, and it is to this task that the most essential part of
the following analysis is devoted. In my first treatment of this
subject I had expressed S, by definition, as a simple function of U
without further foundation, and I was satisfied to show that this from
of entropy meets all the requirements imposed on it by thermodynamics.
At that time I believed that this was the only possible expression and
that consequently Wein's law, which follows from it, necessarily had
general validity. In a later, closer analysis,6 however, it
appeared to me that there must be other expressions which yield the
same result, and that in any case one needs another condition in order
to be able to calculate S uniquely. I believed I had found such a
condition in the principle, which at the time seemed to me perfectly
plausible, that in an infinitely small irreversible change in a system,
near thermal equilibrium, of N identical resonators in the same
stationary radiation field, the increase in the total entropy SN = NS with which it is associated depends only on its total energy UN
= NU and the changes in this quantity, but not on the energy U of
individual resonators. This theorem leads again to Wien's energy
distribution law. But since the latter is not confirmed by experience
one is forced to conclude that even this principle cannot be generally
valid and thus must be eliminated from the theory.7
Thus another condition must now be introduced which will allow
the calculation of S, and to accomplish this it is necessary to look
more deeply into the meaning of the concept of entropy. Consideration
of the untenability of the hypothesis made formerly will help to orient
our thoughts in the direction indicated by the above discussion. In the
following a method will be described which yields a new, simpler
expression for entropy and thus provides also a new radiation equation
which does not seem to conflict with any facts so far determined.
I. CALCULATIONS OF THE ENTROPY OF A RESONATOR AS A FUNCTION OF ITS ENERGY
§1. Entropy depends on disorder and this disorder, according to
the electromagnetic theory of radiation for the monochromatic
vibrations of a resonator when situated in a permanent stationary
radiation field, depends on the irregularity with which it constantly
changes its amplitude and phase, provided one considers time intervals
large compared to the time of one vibration but small compared to the
duration of a measurement. If amplitude and phase both remained
absolutely constant, which means completely homogeneous vibrations, no
entropy could exist and the vibrational energy would have to be
completely free to be converted into work. The constant energy U of a
single stationary vibrating resonator accordingly is to be taken as
time average, or what is the same thing, as a simultaneous average of
the energies of a large number N of identical resonators, situated in
the same stationary radiation field, and which are sufficiently
separated so as not to influence each other directly. It is in this
sense that we shall refer to the average energy U of a single
resonator. Then to the total energy
(Equation #1) UN = NU
of such a system of N resonators there corresponds a certain total entropy
(#2) SN = NS
of the same system, where S represents the average entropy of a single resonator and the entropy SN depends on the disorder with which the total energy UN is distributed among the individual resonators.
§2. We now set the entropy SN of the system
proportional to the logarithm of its probability W, within an arbitrary
additive constant, so that the N resonators together have the energy EN:
(#3) SN = k log W + constant
In my opinion this actually serves as a definition of the
probability W, since in the basic assumptions of electromagnetic theory
there is no definite evidence for such a probability. The suitability
of this expression is evident from the outset, in view of its
simplicity and close connection with a theorem from kinetic gas theory.8
§3. It is now a matter of finding the probability W so that the N resonators together possess the vibrational energy UN. Moreover, it is necessary to interpret UN
not as a continuous, infinitely divisible quantity, but as a discrete
quantity composed of an integral number of finite equal parts. Let us
call each such part the energy element 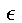 ; consequently we must set
; consequently we must set
(#4) UN = P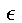
where P represents a large integer generally, while the value of 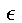 is yet uncertain.
is yet uncertain.
The above paragraph in the original German
Now it is evident that any distribution of the P energy elements
among the N resonators can result only in a finite, integral, definite
number. Every such form of distribution we call, after an expression
used by L. Boltzmann for a similar idea, a "complex." If one denotes
the resonators by the numbers 1, 2, 3, ... N, and writes these side by
side, and if one sets under each resonator the number of energy
elements assigned to it by some arbitrary distribution, then one
obtains for every complex a pattern of the following form:
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10
|
| --------------------------------------
|
| 7 | 38 | 11 | 0 | 9 | 2 | 20 | 4 | 4 | 5
|
Here we assume N = 10, P = 100. The number R of all possible
complexes is obviously equal to the number of arrangements that one can
obtain in this fashion for the lower row, for a given N and P. For the
sake of clarity we should note that two complexes must be considered
different if the corresponding number patters contain the same numbers
but in a different order.
From combination theory one obtains the number of all possible complexes as:
| | N (N+1) (N+2) . . . . (N+P - 1) | | (N+ P - 1) !
|
| R = | --------------------------------------- | = | ----------------
|
| | 1 . 2 . 3 . . . . P | | (N - 1) ! P !
|
Now according to Stirling's theorem, we have in the first approximation:
N ! = NN
Consequently, the corresponding approximation is:
| | ( N + P)N+P
|
| R = | ---------------
|
| | NN . PP
|
§4. The hypothesis which we want to establish as the basis for
further calculation proceeds as follows: in order for the N resonators
to possess collectively the vibrational energy UN, the probability W must be proportional to the number R of all possible complexes formed by distribution of the energy UN
among the N resonators; or in other words, any given complex is just as
probable as any other. Whether this actually occurs in nature one can,
in the last analysis, prove only by experience. But should experience
finally decide in its favor it will be possible to draw further
conclusions from the validity of this hypothesis about the particular
nature of resonator vibrations; namely in the interpretation put forth
by J. v. Kries9 regarding the character of the "original
amplitudes, comparable in magnitude but independent of each other." As
the matter now stands, further development along these lines would
appear to be premature.
§5. According to the hypothesis introduced in connection with
equation (3), the entropy of the system of resonators under
consideration is, after suitable determination of the additive
constant:
| (#5) SN | = k log R
|
| | = k { (N + P) log (N + P) - N log N - P log P}
|
and by considering (4) and (1):
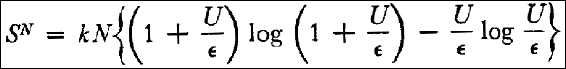
Thus, according to equation (2) the entropy S of a resonator as a function of its energy U is given by:
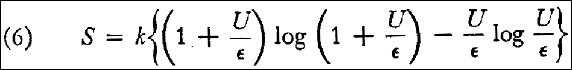
II. Introduction of Wien's Displacement Law
§6. Next to Kirchoff's theorem of the proportionality of
emissive and absorptive power, the so-called displacement law,
discovered by and named after W. Wien,10 which includes as a
special case the Stefan-Boltzmann law of dependence of total radiation
on temperature, provides the most valuable contribution to the firmly
established foundation of the theory of heat radiation, In the form
given by M. Thiesen11 it reads as follows:
E . d =
= 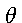 5
5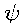 (
(
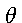 ) . d
) . d
where  is the wavelength , E d
is the wavelength , E d represents the volume density of the "black-body" radiation12 within the spectral region
represents the volume density of the "black-body" radiation12 within the spectral region  to
to  + d
+ d ,
, 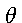 represents temperature and
represents temperature and 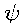 (x) represents a certain function of the argument x only.
(x) represents a certain function of the argument x only.
§7. We now want to examine what Wien's displacement law states
about the dependence of the entropy S of our resonator on its energy U
and its characteristic period, particularly in the general case where
the resonator is situated in an arbitrary diathermic medium. For this
purpose we next generalize Thiesen's form of the law for the radiation
in an arbitrary diathermic medium with the velocity of light c.
Since we do not have to consider the total radiation, but only the
monochromatic radiation, it becomes necessary in order to compare
different diathermic media to introduce the frequency 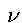 instead of the wavelength
instead of the wavelength  .
.
Thus, let us denote by u d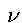 the volume density of the radiation energy belonging to the spectral region
the volume density of the radiation energy belonging to the spectral region 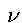 to
to 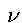 + d
+ d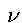 ; then we write: u d
; then we write: u d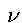 instead of E d
instead of E d ; c /
; c / 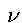 instead of
instead of  , and cd
, and cd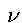 /
/ 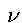 2 instead of d
2 instead of d . From which we obtain
. From which we obtain
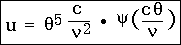
Now according to the well-known Kirchoff-Clausius law, the energy emitted per unit time at the frequency 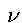 and temperature
and temperature 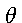 from a black surface in a diathermic medium is inversely proportional to the square of the velocity of propagation c2; hence the energy density U is inversely proportional to c3 and we have:
from a black surface in a diathermic medium is inversely proportional to the square of the velocity of propagation c2; hence the energy density U is inversely proportional to c3 and we have:
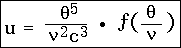
where the constants associated with the function f are independent of c.
In place of this, if f represents a new function of a single argument, we can write:
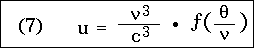
and from this we see, among other things, that as is well known, the radiant energy u .  3 at a given temperature and frequency is the same for all diathermic media.
3 at a given temperature and frequency is the same for all diathermic media.
§8. In order to go from the energy density u to the energy U of a stationary resonator situated in the radiation field and vibrating with the same frequency 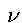 , we use the relation expressed in equation (34) of my paper on irreversible radiation processes13:
, we use the relation expressed in equation (34) of my paper on irreversible radiation processes13:
| | 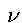 2 2
|
| K = | ------ | U
|
| | c2
|
( K is the intensity of a monochromatic linearly, polarized ray), which together with the well-known equation:
| | 8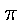 K K
|
| u = | ------ |
|
| | c
|
yields the relation:
| | | 8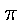 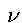 2 2
|
| (#8) | u = | ------ | U
|
| | | c3
|
From this and from equation (7) follows:
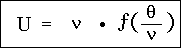
where now c does not appear at all. In place of this we may also write:
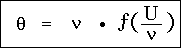
§9. Finally, we introduce the entropy S of the resonator by setting
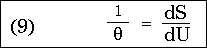
We then obtain:
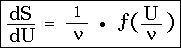
and integrated:
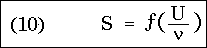
that is, the entropy of a resonator vibrating in an arbitrary diathermic medium depends only on the variable U /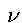 , containing besides this only universal constants. This is the simplest form of Wien's displacement law known to me.
, containing besides this only universal constants. This is the simplest form of Wien's displacement law known to me.
§10. If we apply Wien's displacement law in the latter form to
equation (6) for the entropy S, we then find that the energy element 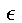 must be proportional to the frequency
must be proportional to the frequency 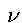 , thus:
, thus:
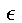 = h
= h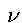
and consequently:
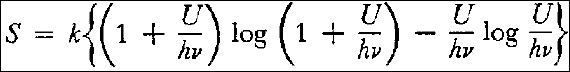
here h and k are universal constants.
By substitution into equation (9) one obtains:
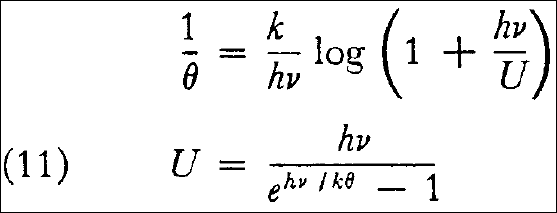
and from equation (8) there then follows the energy distribution law sought for:
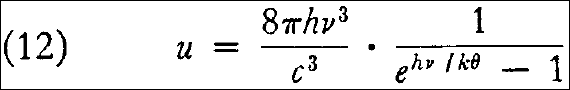
or by introducing the substitutions given in §7, in terms of wavelength  instead of the frequency:
instead of the frequency:
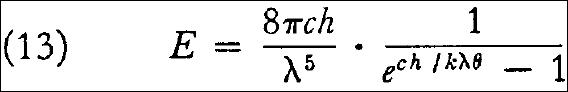
I plan to derive elsewhere the expressions for the intensity and
entropy of radiation progressing in a diathermic medium, as well as the
theorem for the increase of total entropy in nonstationary radiation
processes.
III. Numerical Values
§11. The values of both universal constants h and k may be
calculated rather precisely with the aid of available measurements. F.
Kurlbaum,14 designating the total energy radiating into air from 1 sq cm of a black body at temperature t°C in 1 sec by St, found that:
S100 - S0 = 0.0731 watt/cm2 = 7.31 x 105 erg/cm2 . sec
From this one can obtain the energy density of the total radiation energy in air at the absolute temperature 1:
| 4 . 7.31 . 105
|
| -------------------------- | = 7.061 . 10¯15 erg/cm3 . deg4
|
| 3 . 1010(3734 - 2734)
|
On the other hand, according to equation (12) the energy density of the total radiant energy for 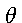 = 1 is:
= 1 is:
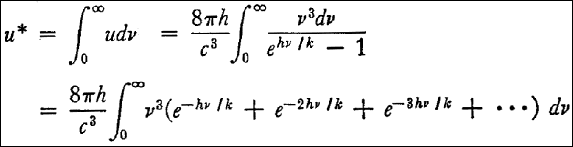
and by termwise integration:
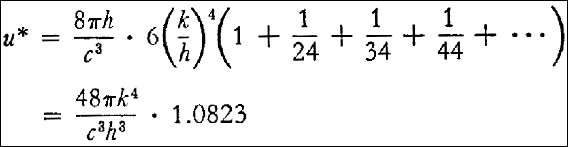
If we set this equal to 7.061 . 10¯15, then, since c = 3 . 1010 cm/sec, we obtain:
| | k4
|
| (#14) | ------ | = | 1.1682 . 1015
|
| | h3
|
§12. O. Lummer and E. Pringswim15 determined the product  m
m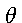 , where
, where  m is the wavelength of maximum energy in air at temperature 0, to be 2940 micron . degree. Thus, in absolute measure:
m is the wavelength of maximum energy in air at temperature 0, to be 2940 micron . degree. Thus, in absolute measure:
 m = 0.294 cm . deg
m = 0.294 cm . deg
On the other hand, it follows from equation (13), when one sets the derivative of E with respect to 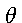 equal to zero, thereby finding
equal to zero, thereby finding  =
=  m
m
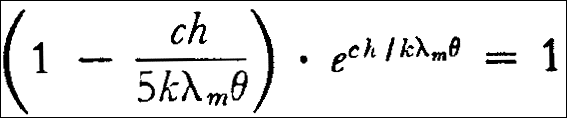
and from this transcendental equation:
 m
m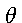 = ch / 4.9651k
= ch / 4.9651k
consequently:
h / k = (4.9561 . 0.294) / 3 . 1010 = 4.866 . 10¯11
From this and from equation (14) the values for the universal constants become:
(#15) h = 6.55 . 10¯27 erg . sec
(#16) k = 1.346 . 10¯16 erg/deg
These are the same number that I indicated in my earlier communication.
Citations
1 O. Lummer and E. Pringsheim, Transactions of the German Physical Society 2 (1900), p. 163
2 H. Rubens and F. Kurlbaum, Proceedings of the Imperial Academy of Science, Berlin, October 25, 1900, p. 929.
3 H. Beckmann, Inaugural dissertation, Tübingen 1898. See also H. Rubens, Weid. Ann. 69 (1899) p. 582.
4 M. Planck, Ann. d. Phys. 1 (1900), p. 719.
5 Compare with equation (8).
6 M. Planck, loc. cit., pp. 730 ff.
7 Moreover one should compare the critiques previously made of this theorem by W. Wien (Report of the Paris Congress 2, 1900, p. 40) and by O. Lummer (loc. cit., 1900, p.92.).
8 L. Boltzmann, Proceedings of the Imperial Academy of Science, Vienna, (II) 76 (1877 ), p. 428.
9 Joh. v. Kries, The Principles of Probability Calculation (Freiburg, 1886), p. 36.
10 W. Wien, Proceedings of the Imperial Academy of Science, Berlin, February 9, 1893, p. 55.
11 M. Thiesen, Transactions of the German Physical Society 2 (1900), p. 66.
12 Perhaps one should speak more appropriately of a "white"
radiation, to generalize what one already understands by total white
light.
13 M. Planck, Ann. D. Phys. 1 (1900), p. 99.
14 F. Kurlbaum, Wied. Ann. 65 (1898), p. 759.
15 O. Lummer and Pringsheim, Transactions of the German Physical Society 2 (1900), p. 176.
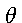 , the dependence of the energy U on the temperature
, the dependence of the energy U on the temperature 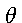 , and since the energy is also related to the density of radiation at the corresponding frequency by a simple relation,5
one also obtains the dependence of this density of radiation on the
temperature. The normal energy distribution is then the one in which
the radiation densities of all different frequencies have the same
temperature.
, and since the energy is also related to the density of radiation at the corresponding frequency by a simple relation,5
one also obtains the dependence of this density of radiation on the
temperature. The normal energy distribution is then the one in which
the radiation densities of all different frequencies have the same
temperature.
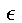 ; consequently we must set
; consequently we must set
 =
= 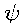 (
(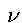 instead of the wavelength
instead of the wavelength 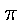 K
K